Treatment of acquired nasolacrimal duct obstruction
The purpose of this leaflet is to provide the patient with information about acquired nasolacrimal duct obstruction and different treatment options.
Symptoms, diagnosis and differential diagnosis
If the nasolacrimal duct or the tear duct becomes blocked, tears cannot flow along their intended path through the nose down into the throat, causing the overflow of tears. As a result, excessive tearing usually occurs both indoors and outdoors. Tearing is also present in dry eye syndrome, from which nasolacrimal duct obstruction must be distinguished. Nasolacrimal duct obstruction is usually characterised by mucoid or purulent discharge from the eye. It can be confirmed by applying pressure on the lacrimal sac. Sometimes, antibiotic drops and massage performed by the patient may help. However, if treatment fails, nasolacrimal duct obstruction can develop into chronic or acute inflammation of the lacrimal sac or dacrocystitis. In this case, a palpable lump appears in the area around the lacrimal sac in the inner corner of the eye, which no longer disappears when pressed on. Symptoms of acute dacrocystitis include redness, pain, and fever.
To diagnose nasolacrimal duct obstruction, probing of the nasolacrimal duct must be performed., anaesthetic drops are instilled in the patient's eye and a probe is inserted into the nasolacrimal duct through either the upper or lower punctum. A saline solution is then flushed through the drainage system to determine the location of the obstruction and the treatment required. It is sometimes possible to remove the blockage during probing.
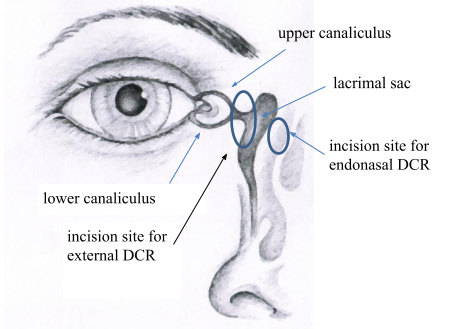
Figure 1. Anatomy of the lacrimal drainage system and incision sites
Treatment options for nasolacrimal duct obstruction
1. Intubation of the nasolacrimal duct
Silicone intubation for obstruction of the nasolacrimal duct is the primary treatment after failed probing. As the surgery is performed under general anaesthesia, patients are advised not to eat or drink on the morning of the surgery. Regular medications should be taken with a sip of water. During the surgery, the lacrimal system is probed to open any blockages and a silicone tube is placed the nasolacrimal duct. If the silicone tube cannot be passed through both canaliculi, only one canaliculus is intubated. The surgery lasts from half an hour to one hour, depending on the anatomy of the patient and the severity of the obstruction. Because the silicone tubes pass through the nose, a postoperative nosebleed may occur, so a nasal pack is usually put in nose. The patient is discharged from the hospital on the same day. In the postoperative period, eye drops and nasal solution prescribed by the doctor should be administered and vigorous blowing of the nose should be avoided. Should the silicone tube come out of the nose, the patient should stay calm and push the tube back into the nose. Rubbing of the inner corner of the eye should be avoided to prevent the displacement of the silicone tube. The silicone tube is usually removed from the nasolacrimal duct in 6–8 weeks. To do this, the doctor calls the patient for an appointment. The doctor cuts through the tube and the patient blows it out in the office. If for some reason this does not work, the tube must be removed using an endoscope. If symptoms persist, another surgery must be performed as described in the next section. Usually, intubation of the nasolacrimal duct is effective in children and young adults, and in patients without a history of inflammation of the lacrimal sac.
2. External dacryocystorhinostomy (external DCR)
If the patient has a history of inflammation of the lacrimal sac or recurrence of the nasolacrimal duct obstruction after intubation, external DCR is performed under general anaesthesia. External DCR involves making a 1 cm incision on the side of the nose to create a new passageway between the lacrimal sac and the nasal cavity by means of osteotomy (Figure 1). This opening is covered with the lacrimal sac and nasal mucosa. A silicone tube is temporarily placed in the nasolacrimal duct to prevent scarring. Since the opening is made in the nasal cavity, bleeding from the nose occurs during and after the surgery. Therefore, nasal packs are placed in the nose. Due to the risk of nosebleed, we recommend patients to spend the night in the hospital so that appropriate treatment can be provided. Patients who take blood thinners, especially Marevan, should definitely inform their doctor. Aspirin should be discontinued five days before the surgery. In the case of Marevan, another medication should be taken as recommended by your family physician. Blood pressure medications must be taken in the morning, although eating and drinking on the morning of the surgery is prohibited. During the surgery, the incision is closed with sutures, which must be removed in7–10 days by a family physician, a treating ophthalmologist or a surgeon. According to different data, the success rate of external DCR is 95%. The downside of external DCR is a scar on the side of the nose, which disappears with time. There may also be bruising around and under the eye, which resolves on its own. After the surgery, eye drops and nasal solution will be prescribed by the doctor. Vigorous blowing of the nose should be avoided for two weeks. The silicone tube should be removed in 6–8 weeks in an outpatient clinic.
3. Endonasal dacryocystorhinostomy (endonasal DCR)
Endonasal DCR involves establishing a connection between the lacrimal sac and the nasal cavity using an endoscope. No incision is made in the skin. To prevent scarring, a silicone tube is temporarily placed in the nasolacrimal duct (Figure 1) which is removed in 6–8 weeks in an outpatient clinic. After the surgery, bleeding from the nose is common; therefore, nasal packs are placed in the nose. Patients can choose whether or not to stay in the hospital overnight. In rare cases, however, the surgery cannot be performed via an endonasal approach due to the anatomy of the patient, and an incision in the skin must be made. The main advantage of endonasal DCR is faster recovery. As no skin incision is made, the mechanism of the lacrimal pump is preserved and no scar remains. External DCR and endonasal DCR have similar success rates. Endonasal DCR is not suitable for patients with various disorders of the nose (polyps, deviated septum) or if, for example, a malignancy is suspected. The doctor will decide which treatment is right for the patient. Possible complications of the surgery include cerebrospinal fluid leakage, and damage to the eyeball and eye muscle, which are fortunately very rare.
Postoperative care
In the first days after the surgery, there may be bleeding from the nose, which stops on its own and is not a cause for concern. It is important to avoid nose blowing for two weeks. After the surgery, eye drops prescribed by the doctor should be instilled into the eye that has been operated on for five weeks. In addition, nasal spray available over the counter in a pharmacy must be used for seven days to reduce swelling, after which the nose can be rinsed with saline solution. Bruising around the area of the surgery is very common and does not require any treatment.
A silicone tube (Figure 2) remains in the nasolacrimal duct for about 6–8 weeks. The tube is removed in a doctor's office, where the doctor cuts it and the patient blows it out of the nose.
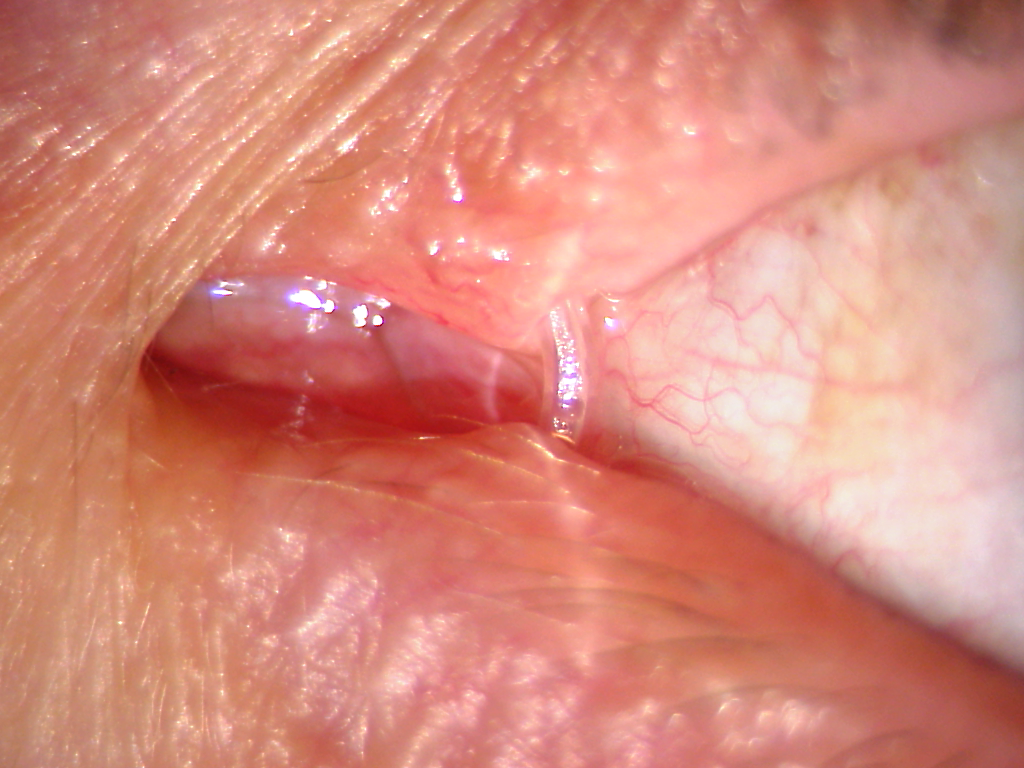
Figure 2. A silicone tube in the nasolacrimal duct.
In children and, in certain special cases, adults, the tube is removed during another surgery. The silicone tube may come out of the nose when the patient sneezes or blows their nose. This is not dangerous and the tube should be pushed back into the nose. Patients should not cut or remove the tube themselves, as part of the tube may remain in the nasolacrimal duct. If a patient is confused and is unable to assess the situation, they can, in extreme cases, seek emergency eye care at East Tallinn Central Hospital.
ITK1047
This information material has been approved by the Eye clinic on 01.01.2025.
 Terviseportaal
Terviseportaal